What Is The Ph Of Rain. The natural acidity of rain is partly balanced by natural bases present in the atmosphere including NH3 emitted by the biosphere and CaCO3 from suspended soil dust. Rain that comes across the continental from the west has a pH of 3848. Typical acid rain has a pH value of 40. It is mildly acidic since it dissolves carbon dioxide CO 2 to form mild carbonic acid.
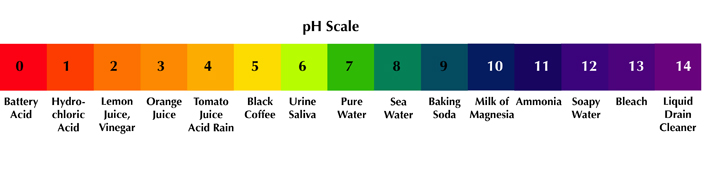
This creates H2CO3 in the raindrops lowering the rains pH value ¹⁷. Rain water is naturally slightly acidic witha pH of about 50. This highly acidic rain is dangerous to plants. The traditional measure of the natural pH of rainwater is around 57. On Americas East Coast rain that is derived from the Atlantic Ocean typically has a pH of 5056. Answer verified by Toppr.
Rain water is naturally slightly acidic witha pH of about 50.
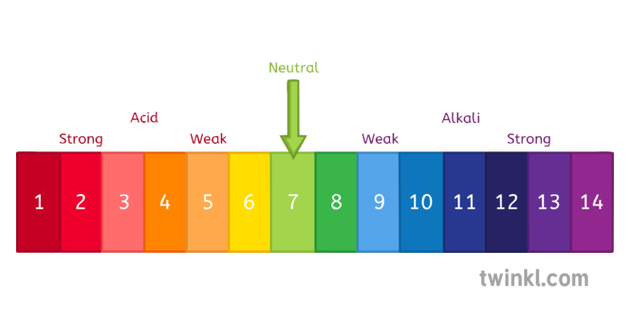
Typical acid rain has a pH value of 40. Acid rain generally has a lower and thus more acidic pH in the range of 40-44. A pH level of 565 though acidic is not considered acid rain. When the pH is above 70 the water is alkaline or basic there are more hydroxide ions than hydrogen ions. PH paper test of rain water and tap water. The natural acidity of rain is partly balanced by natural bases present in the atmosphere including NH3 emitted by the biosphere and CaCO3 from suspended soil dust.