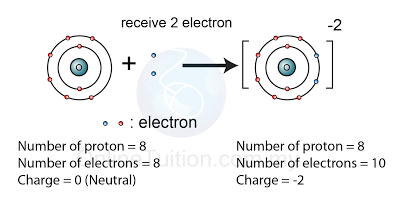
What Is An Oxide Ion. An oxide is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. The electronic configuration of Oxygen is. The ionic formula for calcium oxide is simply CaO. This means it has gained two electrons from another atom.
With the exception of the lighter inert gases helium He neon Ne argon Ar and krypton Kr oxygen O forms at least one binary oxide with each of the elements. What is the electron configuration of the oxide ion o2â. The electronic configuration of calcium ion C a 2 is. Sothis two extra electrons will be attached the last shells orbital 2p. Metal oxides can be compounds that contain alkali metals alkaline earth metals and transition metals. The formulas of the oxide and nitrate ions are respectively.
Metal oxides can be compounds that contain alkali metals alkaline earth metals and transition metals.
It occurs in nature very abundantly and widely distributed. The charge of oxide ion is 2 which means an Oxygen atom gained 2 extra electrons. What is the electron configuration of the oxide ion o2â. O2- refers oxide ion. Any chemical compound that contains O 2- as its anion is also termed an oxide. The formulas of the oxide and nitrate ions are respectively.