
What Is An Ester Bond. A carboxylic acid contains the -COOH group and in an ester the hydrogen in this group is replaced by a hydrocarbon group of some kind. Esters are neutral compounds unlike the acids from which they are formed. Medical Dictionary for the Dental Professions Farlex 2012 Patient discussion about ester. A single bond to a carbon a double bond to an oxygen and a single bond to an oxygen.
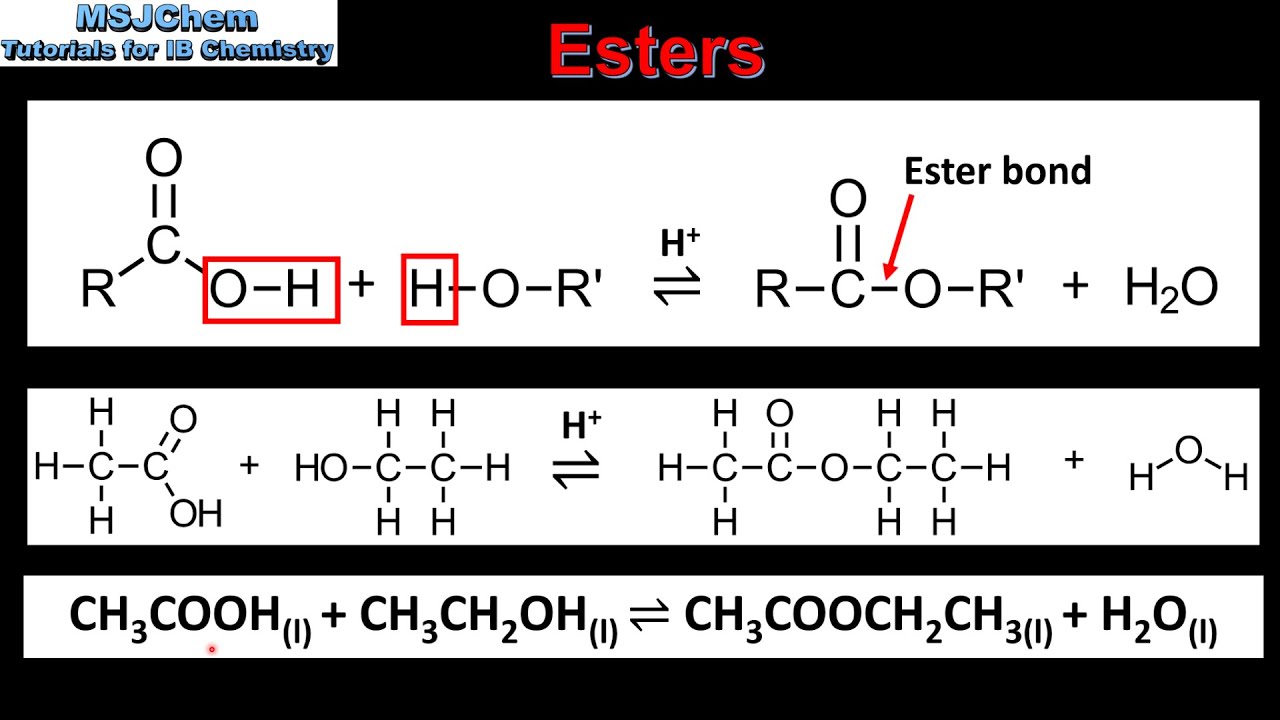
An ester bond is the bond between an alcohol group -OH and a carboxylic acid group -COOH formed by the elimination of a molecule of water H2O. The reaction is known as esterification. Esters feature a carbon-to-oxygen double bond that is also singly bonded to a second oxygen atom. An ester is an organic compound where the hydrogen in the compounds carboxyl group is replaced with a hydrocarbon group. A carboxylic acid contains the -COOH group and in an ester the hydrogen in this group is replaced by a hydrocarbon group of some kind. These ester linkages are generally easy to hydrolyze and hence a number of synthetic polyesters are biodegradable.
Medical Dictionary for the Dental Professions Farlex 2012 Patient discussion about ester.
A single bond to a carbon a double bond to an oxygen and a single bond to an oxygen. Esters are a functional group commonly encountered in organic chemistry. Ester bond formation is described as a dehydration synthesis reaction. Ester names are derived from the parent alcohol and the parent acid. Ester bond is formed when the carboxyl group of fatty acid combine with the hydroxyl group of glycerol. Ester bonds are hydrolyzed by lipases esterases and cutinases phosphate esters by phosphatases and nitriles are transformed by nitrile hydratase and amidase or nitrilases.