
Properties Of O2 Gas. Oxygen is the most widely occurring element on Earth. Chemical Physical and Thermal Properties of Oxygen - O2. Liquid oxygenabbreviated LOx LOX or Lox in the aerospace submarine and gas industriesis the liquid form of molecular oxygenIt was used as the oxidizer in the first liquid-fueled rocket invented in 1926 by Robert H. It is colorless odorless and tasteless.
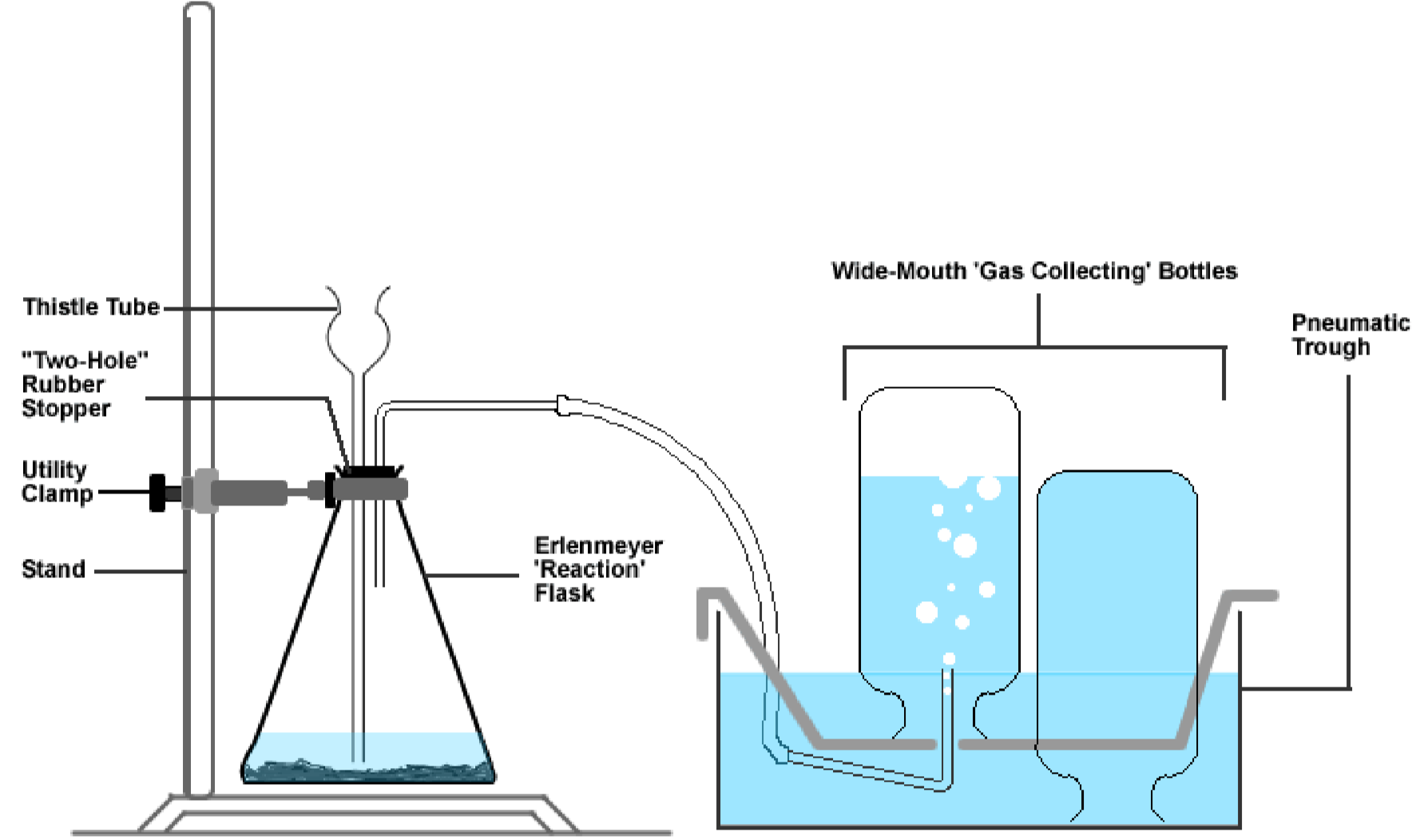
The remaining O2 is combined with various compounds to synthesize cellular structures or elimination products. Properties of Oxygen Gas - Decomposition of Hydrogen Peroxide. The properties of oxygen gas Oxygen gas has a neutral effect on litmus paper It does not burn but helps in burning and it is heavier than the air so It replaces the air It scarcely dissolves in the water it is a colorless tasteless and odorless gas It has the ability to combine directly with most elements forming oxides. Achieve fast response time in safety applications. Most O2 combines with carbon and hydrogen atoms from glucose molecules to form cellular energy known as adenosine triphosphate or ATP along with carbon dioxide CO2 and water. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules O 2 g.
Oxygen exhibits many unique physical and chemical properties.
Properties of Oxygen Gas - Decomposition of Hydrogen Peroxide. Free elemental oxygen occurs naturally as a gas in the form of diatomic molecules O 2 g. It is capable of combining with all elements except the inert gases and is involved especially in combustion processes. To do this heat a small wooden splint with the Bunsen burner until it is glowing. For example oxygen is a colorless and odorless gas with a density greater than that of air and a very low solubility in. Annons Get immediate in situ of O2 gas so you can quickly respond to all possible excursions.