
Human Mass Balance Study. The human mass balance study is one of the most informative studies in the clinical pharmacology package needed for understanding the pharmacokinetic properties of a new chemical entity NCE. An Open-Label Single Center Study to Assess the Absorption Metabolism Excretion and Mass Balance of a Single Oral Dose of 50 mg 90 uCi 14C-HMS5552 in Healthy Adult Male Subjects. Objectives of Human Radiolabeled Mass Balance Studies Human radiolabeled mass balance or AME studies are required by regulatory authorities for the registration of a new drug and therefore are an integral part of the majority of drug development programs. However rarely does the mass balance in radiolabeled excretion studies truly.
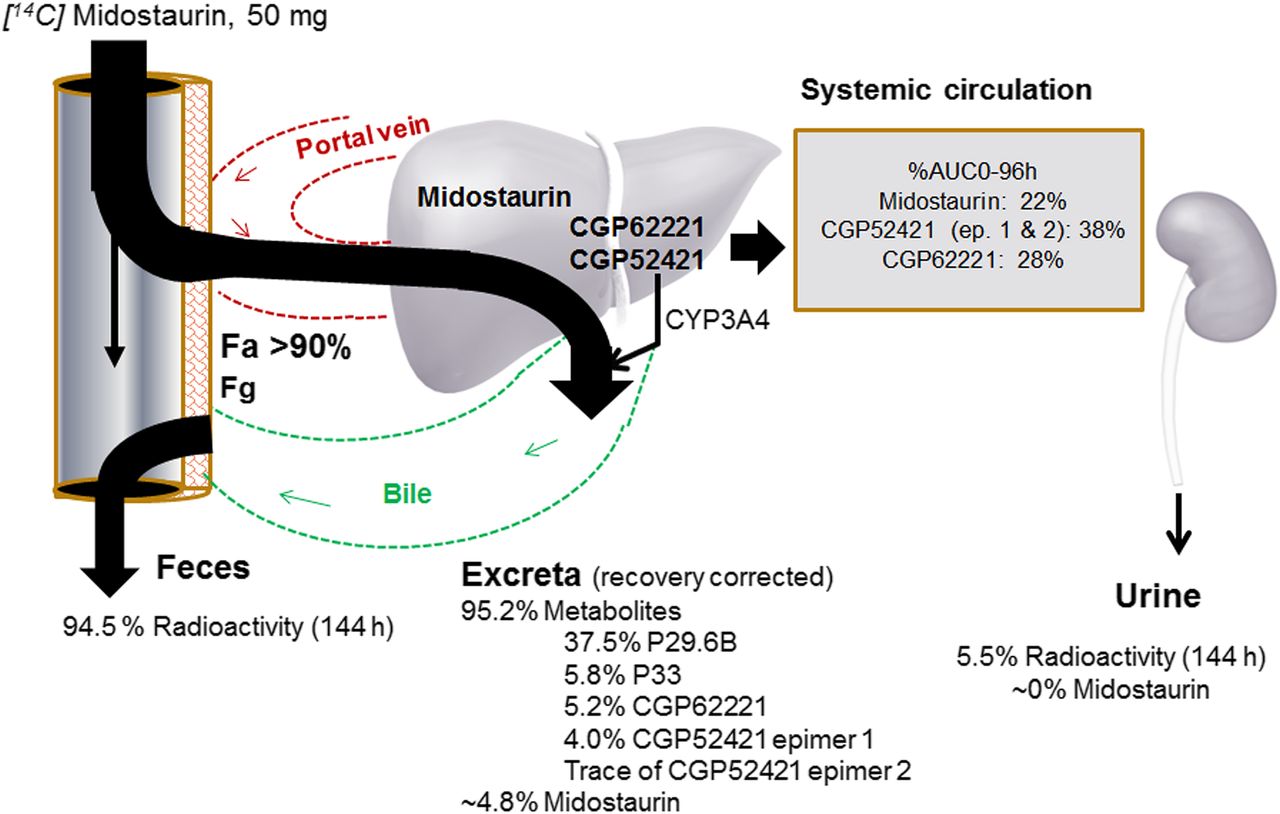
The human mass balance study also often referred to as the ADME study has gathered a lot of attention in the scientific literature and it can be very work intensive and costly and thus is often. However rarely does the mass balance in radiolabeled excretion studies truly. Actual Study Start Date. In addition he will cover formulation of mock batches releasing of a. Actual Primary Completion Date. The human mass balance study is a key study in the Clinical Pharmacology package of new drug applications.
Thus these studies not only determine the rates and routes of excretion but also provide very critical information on the metabolic pathways of drugs in preclinical species and humans.
The human mass balance study ascertains a drugs elimination routes and extent describes time course of elimination elucidates major metabolic pathways identifies and quantifies metabolites and their contribution to the administrated dose. Thus these studies not only determine the rates and routes of excretion but also provide very critical information on the metabolic pathways of drugs in preclinical species and humans. Actual Study Completion Date. The metabolite profiles in laboratory animals and humans are generally accomplished by mass balance and excretion studies in which radiolabeled drugs are administered to these species. Objectives of Human Radiolabeled Mass Balance Studies Human radiolabeled mass balance or AME studies are required by regulatory authorities for the registration of a new drug and therefore are an integral part of the majority of drug development programs. Body weight between 50 and 100 kg and body mass index between 18 and 30 kgm2.