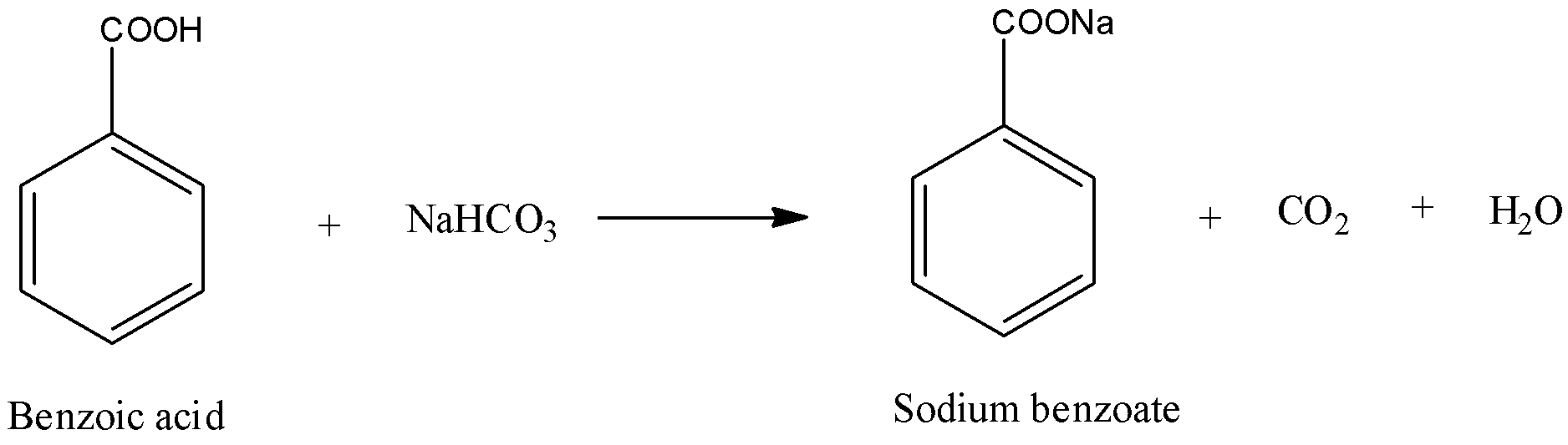
Difference Between Phenol And Benzoic Acid. Phenyl is not a molecule - it does not exist on its own. The name it self differentiate each other phenol is a alcholic Functional group OH and the benzoic acid is a compound with Carboxylic acid group COOH where both of them can be derived frm the benzene derivatives. In summary the key difference between salicylic acid and benzoic acid is that salicylic acid has a OH group ortho to the carboxylic acid group whereas benzoic acid has no OH groups in its structure. It reacts with sodium hydrogen carbonate to give effervescence of carbon dioxide gas.

In fact all carboxylic acids are more acidic than phenols. Answer verified by Toppr. Iv Benzoic acid and Ethyl benzoate can be distinguished by sodium bicarbonate test. Lesser the pKa greater is the acidity. Benzoic acid and phenol both are insoluble in water due to non-polar benzene ring but these molecules become soluble if we react them with an aqueous solution of NaOH which forms water-soluble sodium salt of the benzoic acid Sodium benzoate and the sodium salt of phenol. Phenol is the simplest aromatic alcohol.
Lesser the pKa greater is the acidity.
Here it can be seen that the phenol reacts with the ferric chloride and Iron phenol complex which is violet in colour whereas the benzoic acid does not give this test. The molecular formula of phenol is C 6 H 5 OH. They do not react with weak bases such as sodium hydrogen carbonate. Die Summenformel von Phenol ist C. Benzoic acid and phenol both are insoluble in water due to non-polar benzene ring but these molecules become soluble if we react them with an aqueous solution of NaOH which forms water-soluble sodium salt of the benzoic acid Sodium benzoate and the sodium salt of phenol. Phenol reacts with neutral FeCl3 to form an iron-phenol complex giving violet colouration.